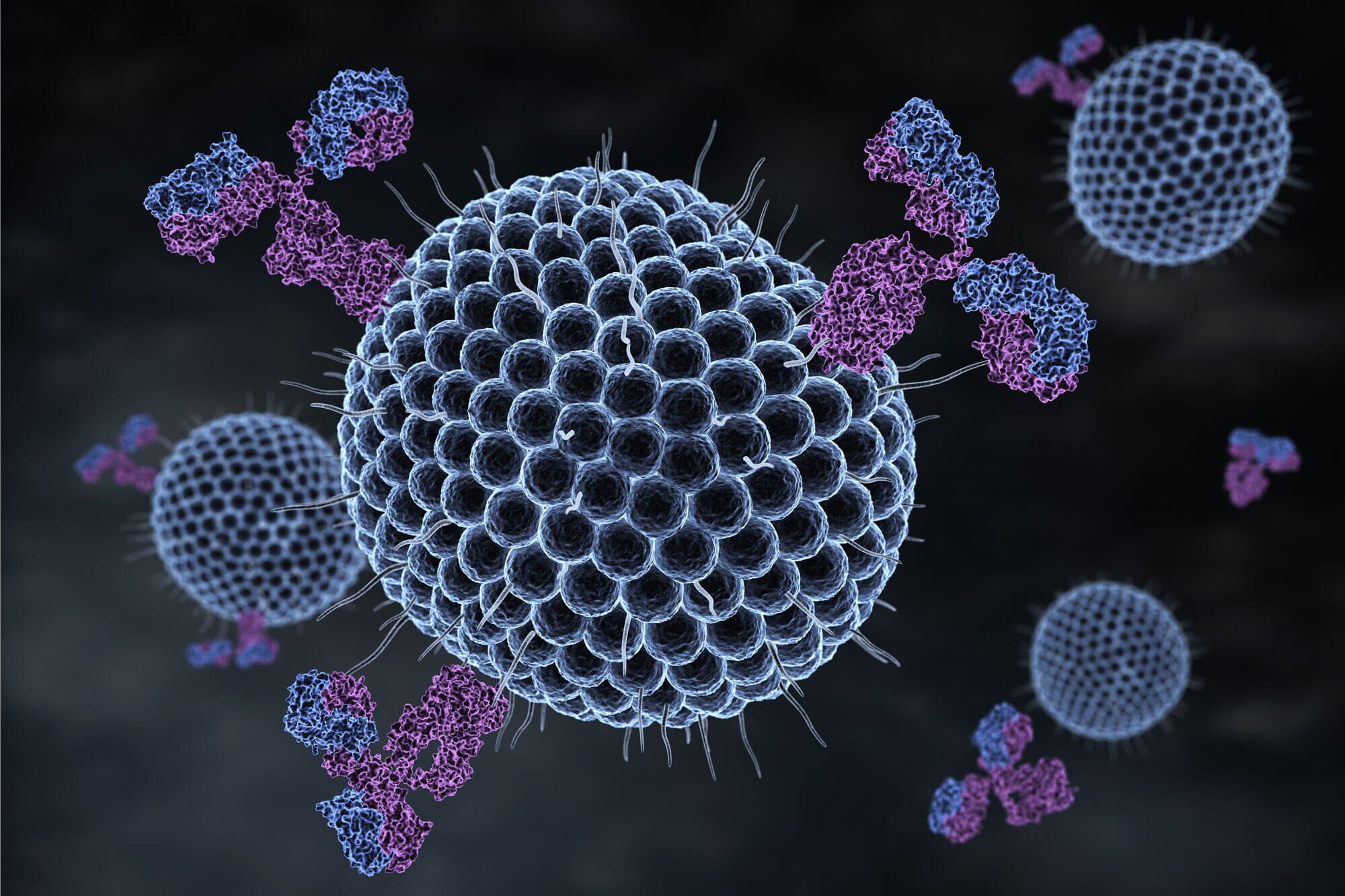
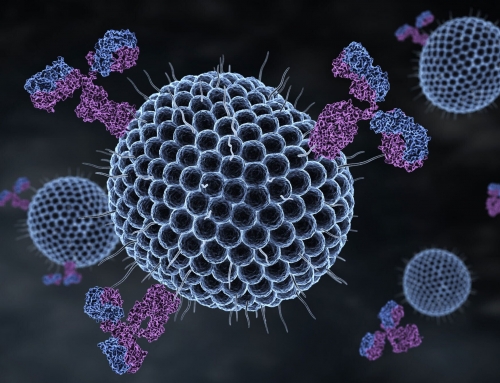
Viruses are appealing vectors for gene delivery because they have evolved over time to deliver nucleic acid directly to specific cells. The same traits that make viruses difficult to treat—their efficiency at targeting particular cells and their ability to avoid immunosurveillance—make them excellent candidates for gene delivery in both research and clinical settings.
However, the complexities of gene therapy and research mean that a number of different viruses have been modified for gene delivery. Different types of viruses may be better suited to different applications.
Some of the most common types of viral vectors for gene delivery are retroviruses, adenoviruses, adeno-associated viruses (AAVs), and the herpes simplex virus. All of these have been altered under laboratory conditions to make them safe and effective gene delivery vehicles.
Retroviruses are a mainstay of current gene therapy and gene delivery.
 Retroviruses replicate by inserting a copy of their genome into the DNA of host cells. Some, like the Moloney murine leukaemia virus (MoMLV) can only deliver genes to non-dividing cells, while others such as vectors derived from HIV can infect both dividing and non-dividing cells alike.
Retroviruses replicate by inserting a copy of their genome into the DNA of host cells. Some, like the Moloney murine leukaemia virus (MoMLV) can only deliver genes to non-dividing cells, while others such as vectors derived from HIV can infect both dividing and non-dividing cells alike.
This means that the particular retrovirus that is used for gene delivery will depend on the type of cells being targeted and the end goals of the therapy. However, their comparative stability makes retroviruses a popular choice for many gene therapy applications.
Most retroviral vectors are replication-defective, meaning that the regions of the virus needed for replication have been removed or replaced with other genes. These retroviruses are able to infect a target cell and deliver a genetic payload, but they do not continue to replicate, as an unmodified virus would.
Lentiviruses, a subset of retroviruses, can infect non-dividing cells because the viral genome is reverse-transcribed when the virus enters the cell. This makes lentiviruses useful as viral vectors for certain kinds of gene delivery, but all lentiviral vectors are replication-defective for the safety of the recipient.
Lentiviruses are produced by transfecting plasmids into a packaging cell line to encode the virion proteins and the reverse transcriptase. A separate plasmid contains the genetic material that will be delivered to the target cell.
Adenoviruses and adeno-associated viruses offer safety in limited gene delivery applications.
 Adenoviruses don’t integrate into the genome of the host cell, which means that adenoviruses are not replicated during cell division. This makes them particularly safe for certain in vitro and in vivo experiments, but limits their broader applications, though they are often used in gene therapy and vaccinations.
Adenoviruses don’t integrate into the genome of the host cell, which means that adenoviruses are not replicated during cell division. This makes them particularly safe for certain in vitro and in vivo experiments, but limits their broader applications, though they are often used in gene therapy and vaccinations.
Most humans have come into contact with adenoviruses, which cause everything from respiratory ailments to eye infections. Therefore, most individuals already have antibodies which can neutralize the virus before it reaches the target cell to deliver its genetic payload.
Because of this, researchers have begun looking into the use of adenoviruses that infect other organisms to use as viral vectors, since humans wouldn’t have antibodies for them already. Another option are adeno-associated viruses or AAVs.
On their own, AAVs are not known to cause disease and trigger only a very mild response from the recipient’s immune system. What’s more, AAVs replicate without incorporation into the chromosome, making them an attractive option for viral vectors. The downside to AAVs is that they are particularly small viruses, and can only carry a small amount of genetic information.
Researchers have also created a modified form of AAV known as a self-complementary adeno-associated virus, which allows for faster expression in the target cell.
The herpes simplex virus (HSV) has been used as a viral vector for gene delivery to the nervous system.
 One of the advantages of the herpes simplex virus is its significant transport capacity. HSV can carry up to ten times the amount of genetic information that can be transported in a adeno-associated virus, for example. However, in its native state, the herpes simplex virus is toxic.
One of the advantages of the herpes simplex virus is its significant transport capacity. HSV can carry up to ten times the amount of genetic information that can be transported in a adeno-associated virus, for example. However, in its native state, the herpes simplex virus is toxic.
When HSV is used as a viral vector for gene delivery, a portion of the genome is usually deleted, rendering the virus unable to duplicate beyond a certain point. HSV can also enter what’s known as an episomal state, which allows the virus to continue expression over a long period of time.
HSV is capable of infecting a wide range of different cells and passing across synapses, which makes it ideal for use in gene delivery to the nervous system—at least, for certain applications.
These are just a few of the viral vectors that have been developed for gene delivery—and a few of the applications in which they are used. Several other vectors have been deployed or are currently being actively researched, while existing vectors are fine-tuned and explored for use in different fields and for different applications.
Regardless of which viral vector is being used and why, one of the key factors in gene delivery is always finding the best possible tool for the job. This requires staying on top of the latest work within the field, since new breakthroughs are being made every day.